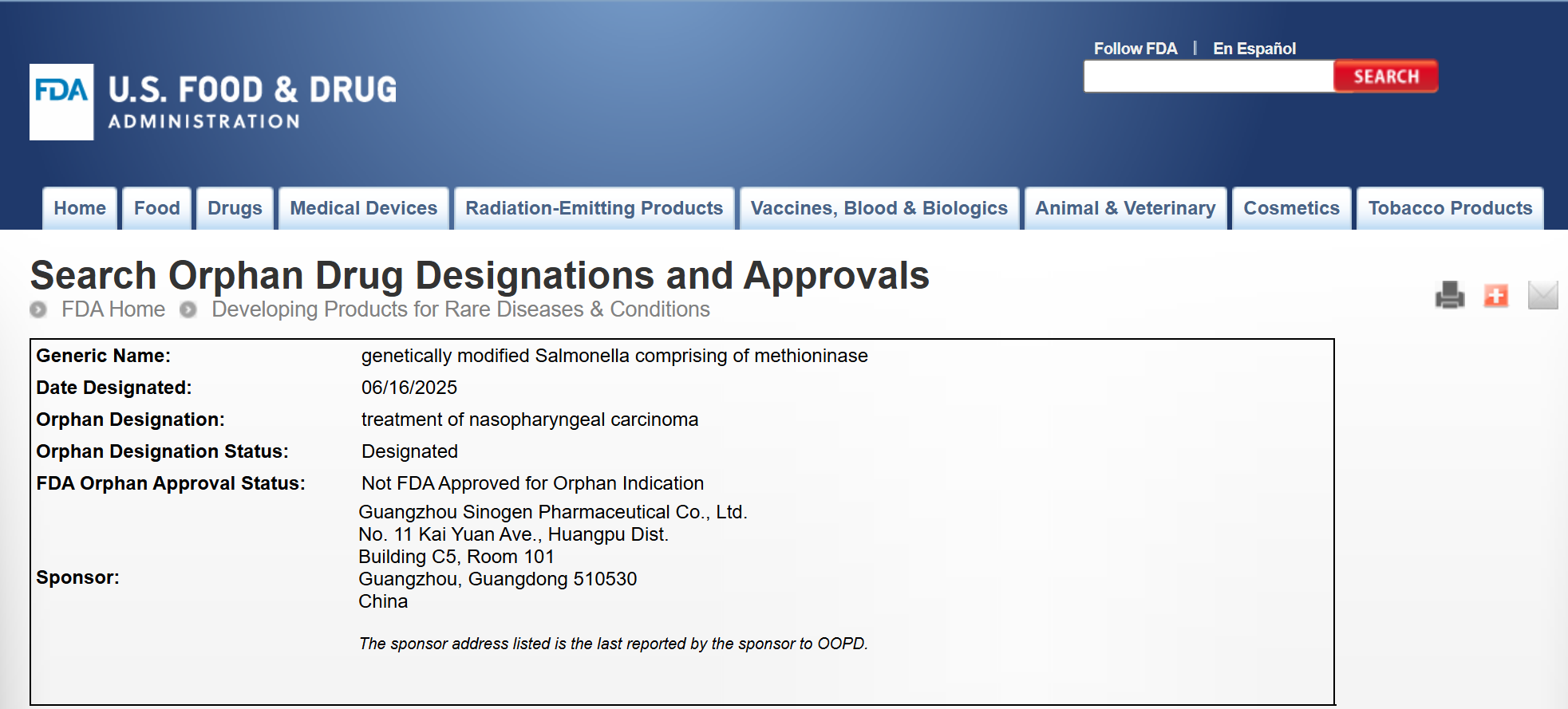
Oncolytic Bacteria SalMet-Vec® Granted Orphan Drug Designation by U.S. FDA for Nasopharyngeal Carcinoma

June 16, 2025 (U.S. Time) – SalMet-Vec®, a leading oncolytic bacterial drug developed by Sinogen Pharmaceutical, has received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of nasopharyngeal carcinoma (NPC). This marks the fifth ODD awarded to SalMet-Vec®, following previous designations for osteosarcoma, hepatocellular carcinoma, small cell lung cancer, and pancreatic cancer.
About Nasopharyngeal Carcinoma (NPC)
Nasopharyngeal carcinoma (NPC) is a malignant epithelial tumor originating from the nasopharyngeal mucosa, primarily affecting the roof and lateral walls (especially the pharyngeal recess) of the nasopharynx. The incidence of NPC varies significantly worldwide, with distinct regional clustering. High-risk areas include:
Southern China (Guangdong, Guangxi, Fujian, Jiangxi)
Southeast Asia (Malaysia, Singapore)
North Africa (Tunisia, Algeria)
In contrast, developed regions such as North America and Europe report much lower incidence rates (<1 per 100,000). China bears the highest global burden of NPC, accounting for 40%–80% of cases worldwide. Southern China (e.g., Guangdong) exhibits an exceptionally high incidence rate of 20–50 per 100,000—10 times higher than northern China and 20 times the global average.
Current immune checkpoint inhibitors used in second-line or later NPC therapies show a limited objective response rate (ORR) of <30%, with median overall survival (OS) for recurrent/metastatic NPC patients at only 16–22 months, highlighting a significant unmet clinical need.
About Sinogen Pharmaceutical
Founded by four national-level leading experts, Sinogen Pharmaceutical is dedicated to developing innovative bacterial vector-based anti-tumor biologics to address global oncology challenges.
SalMet-Vec®, Sinogen’s flagship oncolytic bacterial drug, is the world’s first clinical-stage engineered Salmonella vector designed to deliver and express methioninase, selectively targeting tumors via amino acid metabolism regulation. The company holds full intellectual property rights and global ownership of SalMet-Vec®, which has secured six IND approvals from the U.S. FDA, Taiwan TFDA, and China NMPA.
In clinical studies, SalMet-Vec® has demonstrated strong safety and remarkable efficacy in both intravenous and intratumoral administration across a broad spectrum of solid tumors, including liver cancer, lung cancer, sarcoma, and head and neck cancer.
Regulatory Milestones
FDA Orphan Drug Designations (ODD): Osteosarcoma, hepatocellular carcinoma, small cell lung cancer, pancreatic cancer, and now nasopharyngeal carcinoma.
FDA Fast Track Designations (FTD): Metastatic osteosarcoma and recurrent/metastatic head and neck squamous cell carcinoma.
NMPA Priority Review: Listed as a key product by the Greater Bay Area Division of China’s Center for Drug Evaluation (CDE).
<END>


